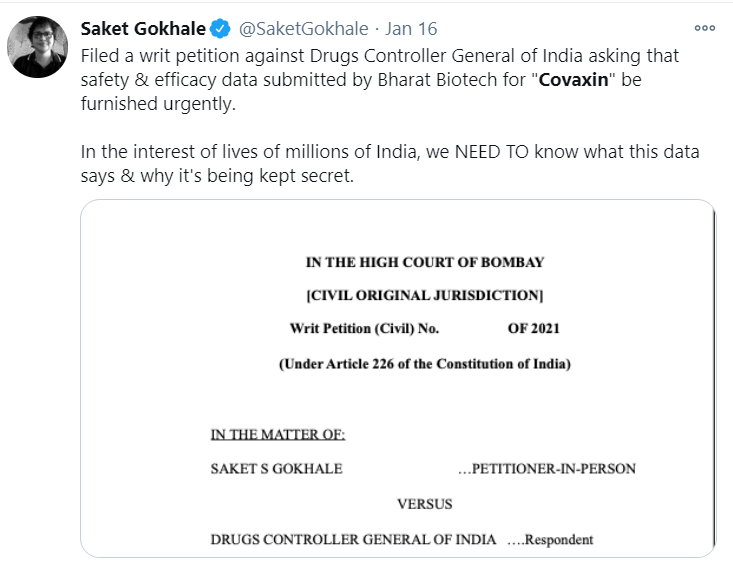
Is COVAXIN Safe ?
India began its vaccination drive against the COVID-19 in January 2021. The event was broadcasted with the director of the AIIMS Dr Sandeep Guleria being administered the shot. However, reports regarding the efficiacy of the vaccine started to surface from all over the country raising serious concerns over its effectiveness and safety. An RTI activist also moved a writ petition before the Bombay High Court seeking information from the Director Controller General of India (DCGI) to reveal to public domain all information on the safety and efficacy of the trial results of Bharat Biotech’s Covaxin, which is the second one to be used alongside the Indian variant of the Oxford vaccine developed by Serum Institute of India.
Resident Doctors of the RML Hospital in Mumbai were also apprehensive about taking COVAXIN, and wrote to the Medical Superintendent earlier this month:
A similar case had struck Bhopal when according to the reports, the participants were kept in the "dark" regarding the vaccine programme. Several developed allergies and one died. Furthermore, the inclusion of the survivors of the erstwhile Bhopal Gas tragedy into the trials raised serious concerns by the activitists and the people:
After over 550 adverse reactions were reported across the country, Bharat Biotech finally issued a statement warning that "those who have weaker immunity or are on a medicine that affects their immune system and people with allergies must not take the Covaxin shot."


The question persists that the COVAXIN is still in the Phase 3 trials, then why are the people made to volunteer for the vaccination drive. The adverse effects range from body ache, headache, fever to nausea and vomiting.
"Additionally, there is a remote chance that the Covaxin could cause a severe allergic reaction. For this reason, your vaccination provider will ask you to stay for 30 minutes after each dose of vaccination at the place where you received your vaccine for monitoring after vaccination," stated Bharat Biotech.
"In case of any serious adverse events, vaccine recipients will be provided with a medically recognised standard of care in the government designated and authorised centres/hospital. The compensation for the serious adverse event will also be provided", it further added.
Vaccination against Covid-19 picked up steam on Monday with 1.48 lakh persons receiving the jab across 25 states and union territories, taking the total vaccinated persons so far to 3.81 lakh. Two deaths were reported. however, government officials dismissed any claims relating the deaths to the vaccine.
There seems to be a hurry to vaccinate people but in the due course, the safety of the vaccine seems to be compromised. Necessary procedures seem to be kept aside which can prove disastrous in the long run and compromise the efficiacy of the vaccine.
To keep yourselves updated with the latest trends, visit Checkbrand.
CATEGORIES
- Digital Marketing
- Marketing
- Entertainment
- Medical
- Science and Technology
- Politics
- Sports
- Environment
- Campaign
- Interview
- Viral
- What's Trending
- Trending News
- Viral Videos
- Youtube Trends
- Social Media Ranking
- Twitter Trends
- Google Trends
- Top Politicians
- Top Cricketers
- Top Influencers
- Best Campaigns
- Google News
- News
-
 Oct 11, 2020
Oct 11, 2020SEO Content Writing Vs. SEO Copywriting:...
-
 Dec 15, 2020
Dec 15, 2020#Karnatakaiphoneplantagitation: Workers...
-
 Dec 15, 2020
Dec 15, 2020#OLA Invests ₹2400 Crores For Our Futur...
-
 Dec 15, 2020
Dec 15, 2020#Snapchat Launches Astrology Profile
-
 Dec 15, 2020
Dec 15, 2020Know Why #BOYCOTTJIOSIM Is Trending On S...
-
 Aug 01, 2023
Aug 01, 2023India's Chandrayaan-3 On Track For Lunar...
-
 May 17, 2023
May 17, 2023Zara Hatke Zara Bachke Trailer Review(Ra...
-
 Aug 04, 2022
Aug 04, 2022'Har Ghar Tiranga' Campaign Created Stor...
-
 Dec 16, 2020
Dec 16, 2020#Skillhaitohfuturehai: Mahindra's Flagsh...
-
 Dec 15, 2020
Dec 15, 2020#OLA Invests ₹2400 Crores For Our Futur...
HIGHLIGHTS
- Realme Pad Specifications Teased, Will C...
- MARKETS: Sensex Down 300 Pts, At Days Lo...
- Afghanistan Crisis Live Updates: NIA Chi...
- Women Will Be Admitted To NDA, "Historic...
- Taliban's New Education Minister Says Ph...
- India's T20 World Cup Selection Question...
- New JioFiber Quarterly Broadband Plans I...
- Explained: How Your Cat Got Its Stripes...
- Who Is Aesha Mukherji? All You Need To K...
- Long Live Test Cricket While We've Virat...