COVID 19: The Battle is far from over
India began its largest vaccine drive on the 16th of January 2021 creating hopes for millions of people and to a country which has been devastated by the global pandemic. But miles away, the shared hope to win the battle against the COVID-19 in in question. Nearly 23 people died in Norway after receiving the first dose of COVID vaccine. Of those deaths, 13 have been autopsied, with the results pointing at the common side effects may have contributed to severe reactions in elderly people, according to the 'Norwegian Medicines Agency'.
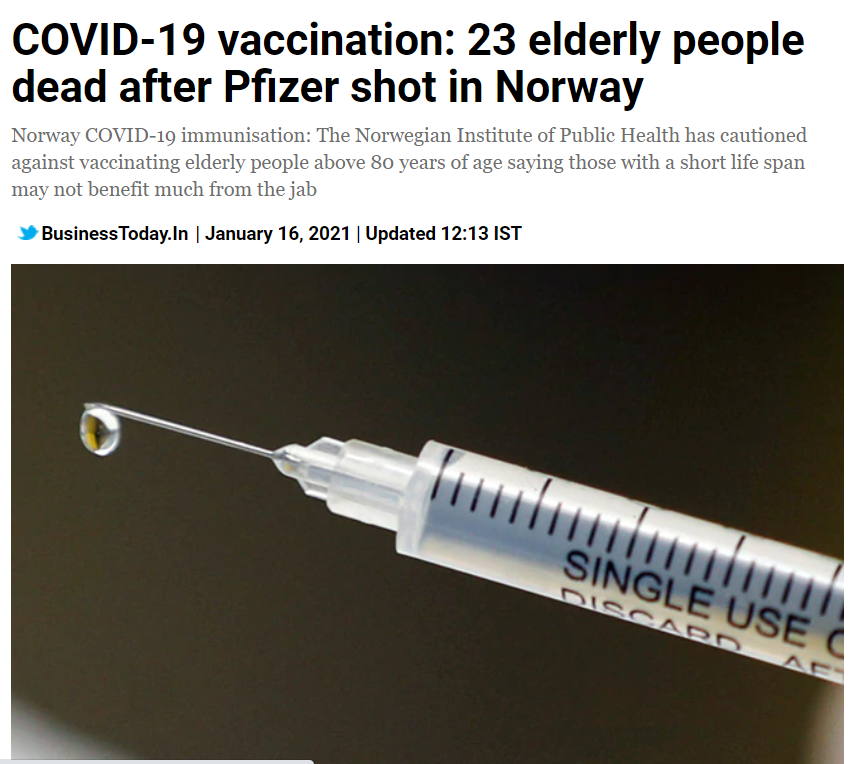
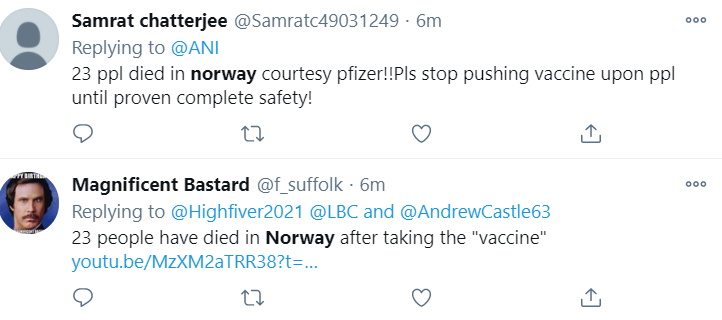
It was quick to garner impressions on the social media platforms:
In a public statement, the Norwegian Institute of Public Health said : “For those with the most severe frailty, even relatively mild vaccine side effects can have serious consequences". It further added: “For those who have a very short remaining life span anyway, the benefit of the vaccine may be marginal or irrelevant.”
Pfizer and BioNTech are working with the Norwegian regulators to investigate the deaths in Norway. The agency found that “the number of incidents so far is not alarming, and in line with expectations."
Allergic reactions have been rare. According to the reports, in the United States, authorities reported 21 cases of severe allergic reactions after administration of about 1.9 million initial doses of the vaccine which puts the incidence at 11.1 cases per million doses.
But what needs to be taken into consideration is the fact that most of the administered doses were on people aged in their early 50's.
The stats however have been fruitful so far in many regards:
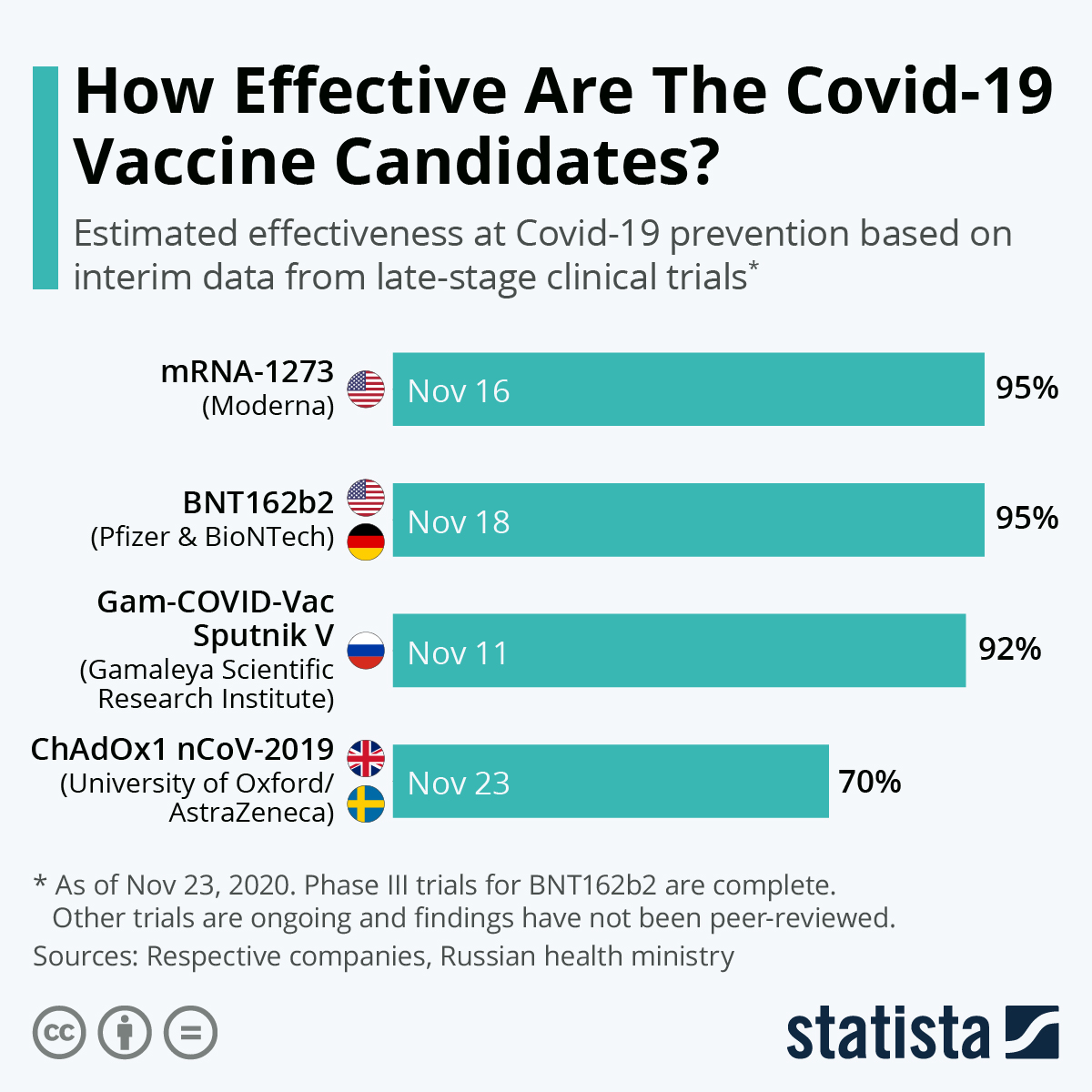
Earlier in France, a person died within two hours of the vaccination. However, a further investigation revealed that the death had no link with the vaccine being administered.
The first survey report of the vaccine in Europe is due to the end of January which will reveal the true efficiacy of the vaccine.
One fact that cannot be contradicted is there seems to be a hurry to implement the vaccination programme.The focus seems to be on rapid development alone, fired by unprecedented political, popular and financial pressures while irreparably damaging public confidence. Even India's vaccination drive has been questioned regarding transparency and the implementation of necessary procedure.
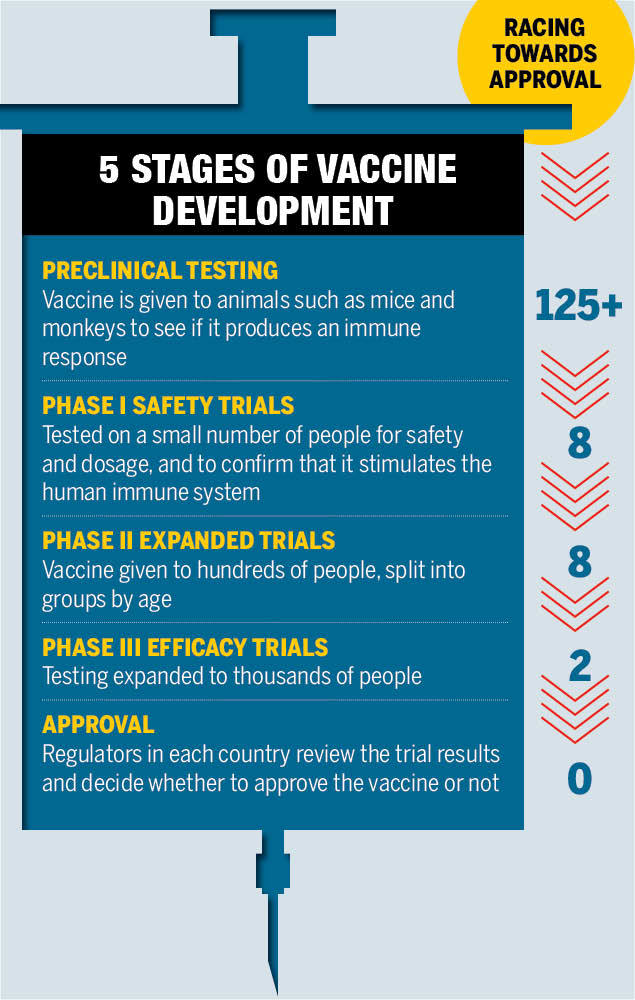
When scientists began the development of a vaccine for the COVID-19 in the early 2020, they did not issue a sooner date since the fastest any vaccine had previously been developed, from sampling to approval, was four years, for mumps in the 1960s. But by December 2020, several giants had begun trial of the COVID vaccine.
Dan Barouch, director of the Center for Virology and Vaccine Research at Harvard Medical School in Boston, Massachusetts stated: “It shows how fast vaccine development can proceed when there is a true global emergency and sufficient resources. It has shown that the development process can be accelerated substantially without compromising on safety", to substantiate, he cited the use of RNA (mRNA) as a breakthrough development.
In its most recent re- tweet, BioNTech showed the efficiacy of the vaccine:
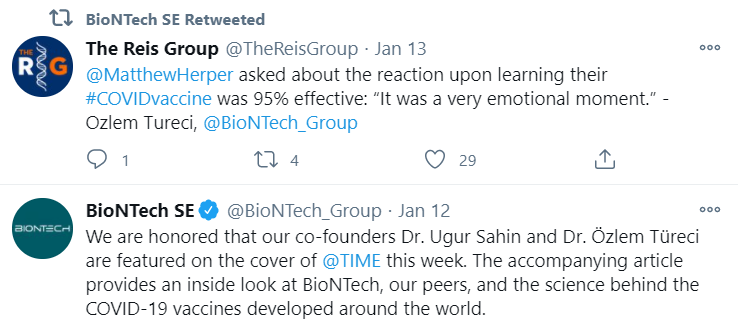
However, many grey areas still exist:
The race to develop a vaccine against the coronavirus seems to being implemented without taking into consideration much precautions and the necessary procedures needed for a vaccine. It reminds of the nuclear or the space race but we forget that here, billions of lives are directly at stake. The future of this battle still remains uncertain. With doubts arising over the safety of the vaccines, mutation of the virus like the recent ones in the UK and South Africa, the battle is still far from over.
To keep yourselves updated with the latest trends and happenings around the globe on a vast array of subjects, visit checkbrand.
CATEGORIES
- Digital Marketing
- Marketing
- Entertainment
- Medical
- Science and Technology
- Politics
- Sports
- Environment
- Campaign
- Interview
- Viral
- What's Trending
- Trending News
- Viral Videos
- Youtube Trends
- Social Media Ranking
- Twitter Trends
- Google Trends
- Top Politicians
- Top Cricketers
- Top Influencers
- Best Campaigns
- Google News
- News
-
 Oct 11, 2020
Oct 11, 2020SEO Content Writing Vs. SEO Copywriting:...
-
 Dec 15, 2020
Dec 15, 2020#Karnatakaiphoneplantagitation: Workers...
-
 Dec 15, 2020
Dec 15, 2020#OLA Invests ₹2400 Crores For Our Futur...
-
 Dec 15, 2020
Dec 15, 2020#Snapchat Launches Astrology Profile
-
 Dec 15, 2020
Dec 15, 2020Know Why #BOYCOTTJIOSIM Is Trending On S...
-
 Aug 01, 2023
Aug 01, 2023India's Chandrayaan-3 On Track For Lunar...
-
 May 17, 2023
May 17, 2023Zara Hatke Zara Bachke Trailer Review(Ra...
-
 Aug 04, 2022
Aug 04, 2022'Har Ghar Tiranga' Campaign Created Stor...
-
 Dec 16, 2020
Dec 16, 2020#Skillhaitohfuturehai: Mahindra's Flagsh...
-
 Dec 15, 2020
Dec 15, 2020#OLA Invests ₹2400 Crores For Our Futur...
HIGHLIGHTS
- Realme Pad Specifications Teased, Will C...
- MARKETS: Sensex Down 300 Pts, At Days Lo...
- Afghanistan Crisis Live Updates: NIA Chi...
- Women Will Be Admitted To NDA, "Historic...
- Taliban's New Education Minister Says Ph...
- India's T20 World Cup Selection Question...
- New JioFiber Quarterly Broadband Plans I...
- Explained: How Your Cat Got Its Stripes...
- Who Is Aesha Mukherji? All You Need To K...
- Long Live Test Cricket While We've Virat...